-
Web sayfası bildirimcisi
- EXPLORE
-
Sayfalar
-
Blogs
-
Forums
Reprocessed Medical Devices Market Size Projected to Reach USD 11.15 Billion by 2032
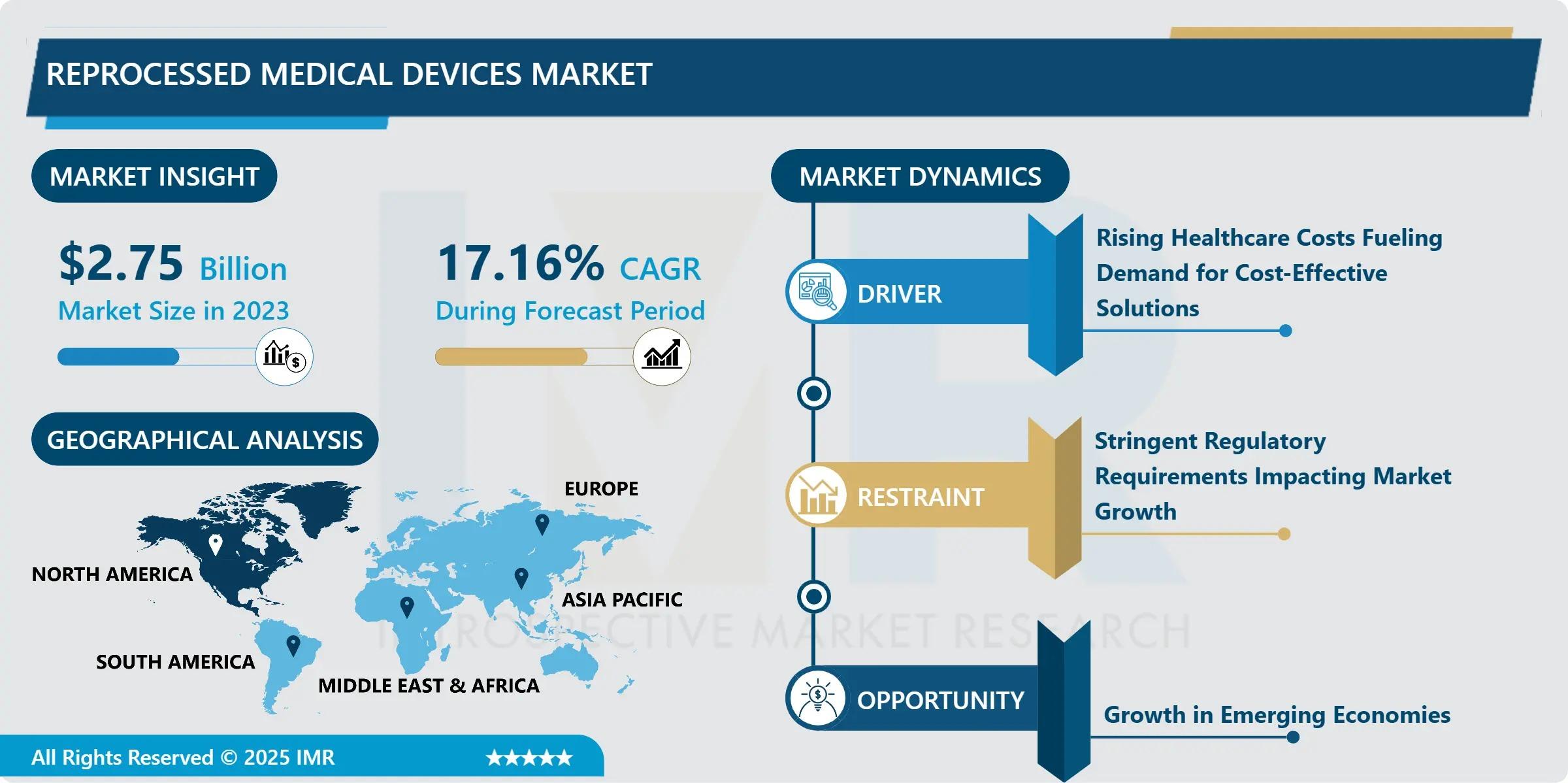
“According to a new report published by Introspective Market Research, Reprocessed Medical Devices Market by Device Type, End User, and Region, The Global Reprocessed Medical Devices Market Size Was Valued at USD 2.75 Billion in 2023 and is Projected to Reach USD 11.15 Billion by 2032, Growing at a CAGR of 17.16% From 2024–2032.”
The reprocessed medical devices market involves the regulated cleaning, sterilization, testing, and reuse of single-use medical devices to ensure they meet original manufacturer safety and performance standards. These devices are widely used across hospitals, ambulatory surgical centers, and specialty clinics to reduce operational costs while maintaining high-quality patient care.
Reprocessed medical devices offer significant advantages over traditional single-use products, including substantial cost savings, reduced medical waste, and enhanced sustainability. Devices such as catheters, laparoscopic instruments, and electrophysiology devices are commonly reprocessed and reused without compromising patient safety.
Rising healthcare expenditure, growing environmental concerns, and increasing acceptance of reprocessing practices by regulatory authorities are driving market expansion globally. As healthcare systems seek cost-effective and eco-friendly solutions, demand for reprocessed medical devices continues to grow rapidly.
Market Segmentation
The Reprocessed Medical Devices Market is segmented into Device Type, End User, and Region.
By Device Type, the market is categorized into (Cardiovascular Devices, Laparoscopic Devices, Orthopedic Devices, General Surgery Devices, and Others).
By End User, the market is categorized into (Hospitals, Ambulatory Surgical Centers, and Clinics).
By Region, the market is categorized into (North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa).
Growth Driver
One of the primary growth drivers of the reprocessed medical devices market is the increasing pressure on healthcare providers to reduce operational costs. Reprocessed devices offer cost savings of up to 30–50% compared to new devices, enabling hospitals to manage budget constraints without sacrificing clinical outcomes. Additionally, regulatory approvals from authorities such as the FDA have enhanced confidence in reprocessed devices, further accelerating adoption across healthcare facilities worldwide.
Market Opportunity
A major market opportunity lies in the growing emphasis on sustainability and waste reduction in healthcare systems. Reprocessed medical devices significantly lower medical waste and carbon footprints, aligning with global environmental initiatives. Emerging economies, particularly in Asia-Pacific and Latin America, present untapped opportunities as healthcare infrastructure expands and awareness regarding cost-effective and sustainable medical solutions increases.
Detailed Segmentation
Reprocessed Medical Devices Market, Segmentation
The Reprocessed Medical Devices Market is segmented on the basis of Device Type, End User, and Region.
Device Type
The Device Type segment is further classified into Cardiovascular Devices, Laparoscopic Devices, and Orthopedic Devices. Among these, the Cardiovascular Devices sub-segment accounted for the highest market share in 2023. This dominance is attributed to the high usage of electrophysiology catheters and compression sleeves in cardiac procedures, coupled with strong regulatory approvals and consistent clinical performance.
End User
The End User segment is further classified into Hospitals, Ambulatory Surgical Centers, and Clinics. Among these, the Hospitals sub-segment accounted for the highest market share in 2023. Hospitals represent the largest adopters due to high surgical volumes, strong purchasing power, and established reprocessing partnerships that enable cost optimization and sustainable operations.
Some of The Leading/Active Market Players Are-
• Stryker Corporation (USA)
• Johnson & Johnson (USA)
• Medtronic plc (Ireland)
• Sterilmed, Inc. (USA)
• Vanguard AG (Germany)
• ReMed, Inc. (USA)
• NEScientific, Inc. (USA)
• Innovative Health, Inc. (USA)
• SureTek Medical, Inc. (USA)
• Centurion Medical Products (USA)
• Arjo AB (Sweden)
• Medline Industries, LP (USA)
• 3M Health Care (USA)
• Boston Scientific Corporation (USA)
and other active players.
Key Industry Developments
In March 2024, several U.S. hospitals expanded partnerships with third-party reprocessing companies to reduce medical supply costs.
This initiative helped healthcare providers achieve significant cost savings while improving sustainability metrics and reducing single-use medical waste.
In September 2023, a leading medical device reprocessing firm received expanded FDA clearance for additional cardiovascular devices.
The approval strengthened market confidence and increased adoption of reprocessed devices across hospitals and surgical centers globally.
Key Findings of the Study
• Cardiovascular devices dominate the market by device type
• Hospitals remain the leading end users globally
• North America holds the largest regional share
• Cost reduction and sustainability are key growth drivers
• Regulatory support is accelerating market adoption
About Us
At Introspective Market Research Private Limited, we are a forward-thinking research consulting firm committed to driving growth in the Reprocessed Medical Devices Market. With deep insights, strategic solutions, and holistic research, we empower businesses to achieve success and dominance in the global healthcare industry.
More Info:- https://introspectivemarketresearch.com/reports/reprocessed-medical-devices-market/
📞 Contact Us
Introspective Market Research Pvt. Ltd.
📞 Phone: +91-91753-37569
📧 Email: sales@introspectivemarketresearch.com
🌐 Web: www.introspectivemarketresearch.com


