-
Web sayfası bildirimcisi
- EXPLORE
-
Sayfalar
-
Blogs
-
Forums
Global Ataxia Market Strategic Analysis and Industry Forecast
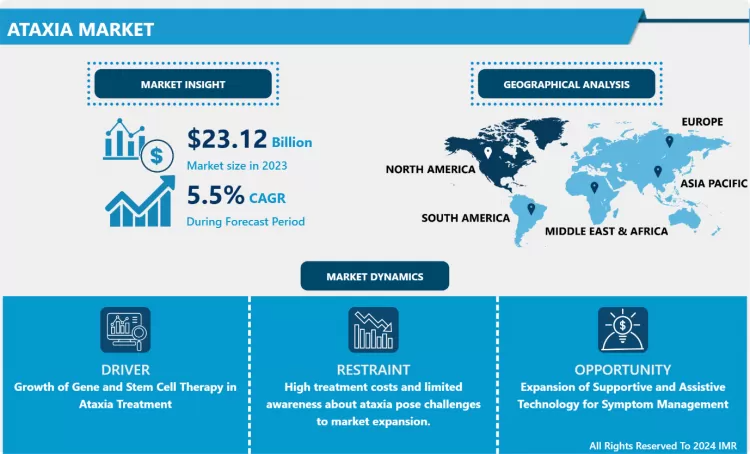
The Global Ataxia Market Size Was Valued at USD 23.12 Billion in 2023 and is Projected to Reach USD 37.43 Billion by 2032, Growing at a CAGR of 5.50% From 2024-2032.
Ataxia is a neurological sign consisting of a lack of voluntary coordination of muscle movements that can include gait abnormality, speech changes, and abnormalities in eye movements. The ataxia market focuses on the development and commercialization of pharmacological treatments, physical therapy aids, and genetic diagnostic tools designed to manage various forms of this condition, including Friedreich's ataxia, spinocerebellar ataxias, and ataxia-telangiectasia. This market is characterized by a high degree of specialization in orphan drug development and neuro-rehabilitative technologies aimed at improving the quality of life for patients with degenerative coordination disorders.
The primary advantage of modern ataxia interventions over traditional supportive care is the move toward disease-modifying therapies and precision medicine. While traditional treatments focused solely on symptom management, the current market is witnessing a shift toward gene therapies and targeted molecular treatments that address the underlying genetic mutations or mitochondrial dysfunctions causing the disease. Major industries and healthcare segments utilizing these solutions include neurology clinics, rehabilitation centers, and specialized genetic research institutes. The rising global prevalence of neurological disorders and the increasing availability of sophisticated diagnostic imaging are key drivers for the market’s steady growth.
👉 To request a sample report: https://introspectivemarketresearch.com/reports/ataxia-market/
Market Segmentation
The Ataxia Market is segmented into Type, Treatment, and End-User. By Type, the market is categorized into (Friedreich’s Ataxia, Ataxia-Telangiectasia, Spinocerebellar Ataxias, Others). By Treatment, the market is categorized into (Pharmacotherapy, Stem Cell Therapy, Physical Therapy, Others). By End-User, the market is categorized into (Hospitals, Specialty Clinics, Research Institutes, Homecare Settings).
Growth Driver
The principal growth driver for the Ataxia Market is the significant breakthrough in orphan drug designations and the increasing investment in neurodegenerative disease research. Because many forms of ataxia are rare or "orphan" diseases, governments in North America and Europe provide substantial R&D incentives, including tax credits and extended market exclusivity, to pharmaceutical companies. This has led to a robust clinical pipeline of innovative therapies that target the Nrf2 pathway and mitochondrial function. Furthermore, the rising awareness among healthcare providers regarding early symptoms and the availability of advanced genetic testing have drastically improved diagnosis rates, fueling the demand for specialized treatment options.
Market Opportunity
A major market opportunity lies in the development of non-invasive neuro-stimulation devices and wearable technology for motor-skill rehabilitation. Ataxia patients often suffer from severe balance and gait issues, and current pharmacological options are often limited in their ability to restore physical coordination. The integration of AI-driven gait-assistive devices and deep brain stimulation (DBS) technologies offers a lucrative niche for med-tech companies. Additionally, the expansion of telemedicine and remote monitoring tools allows for long-term management of ataxia in homecare settings, providing a high-value growth path for digital health innovators focusing on chronic neurological monitoring and physical therapy adherence.
Detailed Segmentation
Title: Ataxia Market Market, Segmentation The Ataxia Market is segmented on the basis of Type, Treatment, and End-User.
Type
The Type segment is further classified into Friedreich’s Ataxia, Ataxia-Telangiectasia, and Spinocerebellar Ataxias. Among these, the Friedreich’s Ataxia sub-segment accounted for the highest market share in 2023. This dominance is due to the relatively higher prevalence of this specific autosomal recessive form of the disease compared to other hereditary ataxias. As it is the most common inherited ataxia, it has attracted the most significant focus from pharmaceutical developers, leading to the recent approvals of first-of-their-kind disease-modifying therapies. The established diagnostic protocols and a highly organized patient advocacy network have also contributed to better clinical data and higher treatment adoption rates within this segment.
Treatment
The Treatment segment is further classified into Pharmacotherapy, Stem Cell Therapy, and Physical Therapy. Among these, the Pharmacotherapy sub-segment accounted for the highest market share in 2023. This is attributed to the widespread use of various drug classes, including antioxidants, nerve protectors, and muscle relaxants, to manage the complex symptoms of ataxia. The segment is currently undergoing a transformative shift as the market transitions from generic symptom-management drugs to high-value, patented orphan drugs. The convenience of oral medications and the increasing success of clinical trials involving small molecule inhibitors make pharmacotherapy the primary revenue generator for the market.
Some of The Leading or Active Market key Players Are-
Biogen Inc. (United States) Reata Pharmaceuticals (United States) PTC Therapeutics (United States) Takeda Pharmaceutical Company Limited (Japan) Sanofi S.A. (France) Bristol-Myers Squibb Company (United States) Pfizer Inc. (United States) AstraZeneca plc (United Kingdom) F. Hoffmann-La Roche Ltd (Switzerland) Novartis AG (Switzerland) Ionis Pharmaceuticals, Inc. (United States) Design Therapeutics (United States) and other active players.
Key Industry Developments
In February 2024, Biogen Inc. finalized the acquisition of Reata Pharmaceuticals, bringing the first FDA-approved treatment for Friedreich’s Ataxia into its neurology portfolio. This development is a landmark for the market, providing a commercial-scale infrastructure for a treatment that specifically targets the oxidative stress pathways in patients. The acquisition signals a consolidated effort by major pharmaceutical players to lead the market in rare neurological disorders.
In May 2024, PTC Therapeutics announced positive top-line results from its Phase 3 clinical trial for a novel pediatric ataxia treatment. This new drug candidate targets mitochondrial biogenesis and has shown significant improvement in motor function for children with rare hereditary coordination issues. The news has accelerated the company's timeline for regulatory filing, potentially introducing a new standard of care for early-onset ataxia management.
Key Findings of the Study
· Dominant Segments: Friedreich’s Ataxia type and Pharmacotherapy treatments remain the primary revenue drivers for the global market.
· Leading Regions: North America leads the market share, supported by advanced genetic testing facilities and robust orphan drug legislative frameworks.
· Key Growth Drivers: Rising investments in orphan drug R&D and improved diagnostic accuracy for neurodegenerative diseases are the main catalysts.
· Market Trends: There is a significant trend toward the adoption of gene therapy and neuro-rehabilitation wearables to address the root causes of motor dysfunction.
🔍 𝐈𝐧-𝐃𝐞𝐩𝐭𝐡 𝐑𝐞𝐩𝐨𝐫𝐭:
https://introspectivemarketresearch.com/reports/ataxia-market/
About Introspective Market Research
Introspective Market Research is a global provider of data-driven market intelligence and strategic advisory services. Our analysts and consultants deliver comprehensive reports, actionable insights and customized consulting to clients across chemicals & materials, healthcare, energy, environment, infrastructure, and advanced manufacturing sectors.
Media Contact:
Introspective Market Research.
Email: press@introspectivemarketresearch.com
Website: http://www.introspectivemarketresearch.com
Phone: +91-91753-37569


